Have you ever wondered why your ice cubes melt when placed in warm water, but the water feels colder? It’s a common belief that ice cools water, but the reality is a little more scientific. In this article, we’ll explore why the water actually heats up the ice rather than the other way around, and how energy transfer plays a crucial role in this process.
Understanding Energy Transfer: From Hot to Cold
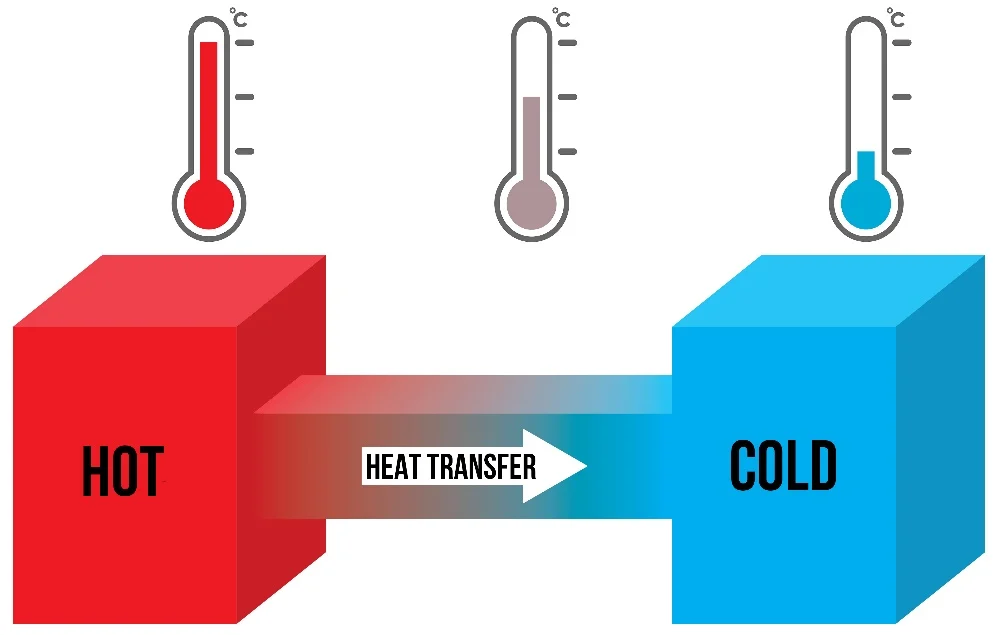
Before diving into the ice and water scenario, let’s quickly cover a fundamental concept in thermodynamics: energy transfer. Energy, in the form of heat, always moves from the hotter object to the colder one. This means that when two objects of different temperatures are in contact, heat will flow from the warmer object to the cooler one until both objects reach thermal equilibrium (a state where both have the same temperature).
So, when you place ice cubes into a glass of warm water, the heat from the water transfers to the ice, causing it to melt. In other words, it’s the warm water that is transferring heat to the cold ice, making the ice melt faster, not the other way around.
What Happens When Ice Meets Warm Water?
When you drop ice into a glass of warm water, the temperature of the water is higher than the temperature of the ice. The warmer water molecules move faster and collide with the colder, slower molecules in the ice. These collisions cause the ice to absorb heat, which causes it to melt. As the ice melts, the water’s temperature decreases because the heat is being absorbed by the ice.
However, it’s crucial to remember that the ice itself isn’t responsible for cooling the water. Rather, it’s the melting process, where the ice absorbs the heat, that causes the water to become cooler. The ice isn’t a cooling agent in the traditional sense—it’s simply a medium for energy transfer.
Video : What happens when you put 3 ice cubes in warm water
Energy Transfer and the Melting Point of Ice
When heat is transferred to ice, it provides the energy needed to break the bonds between the water molecules in the solid ice. This process is called melting. Ice melts at 0°C (32°F) at standard pressure, but when you add heat, the temperature of the ice rises until it reaches its melting point.
Once the ice has absorbed enough heat, it turns into liquid water, and the heat continues to flow into the water. Interestingly, even after the ice melts completely, the water temperature won’t rise immediately. The heat is still being used to transition the ice from solid to liquid. Only once the melting process is complete will the water temperature start to rise.
Why Does Ice Melt Faster in Warm Water?
If you’ve ever noticed that ice melts much faster in warm water than in cold water, this is a perfect demonstration of the principle of energy transfer. The warmer the water, the more energy it has to transfer to the ice, causing it to melt faster. In contrast, cold water has less energy to transfer, so the ice will melt more slowly.
This principle is often used in practical applications. For example, when you place ice into a cooler filled with warm beverages, the ice absorbs heat from the drinks more efficiently, cooling them down quickly.
Does Ice Cool the Drink?
So, if ice isn’t cooling your drink, then what’s happening? The ice is not cooling the liquid directly but instead absorbing heat from it. When the ice absorbs heat, the temperature of the liquid drops, giving the impression that the ice is cooling the drink. However, the ice is merely facilitating the transfer of heat from the warmer liquid to the colder ice. The cooling effect is a result of the heat transfer process, not the ice itself.

In essence, the ice is simply a heat sink that takes in energy from the surrounding liquid. The more ice you add, the more energy is absorbed, which leads to a more noticeable cooling effect. But remember, it’s the water or liquid that heats up the ice, and the ice melts as it absorbs that heat.
Why This Understanding Matters
Grasping the concept of energy transfer and how ice works in cooling liquids isn’t just an interesting fact—it also has real-world applications. For instance, when designing refrigeration systems or cooling devices, engineers must account for how materials like ice absorb and release heat. Understanding these processes can lead to more efficient cooling solutions.
Additionally, knowing the dynamics of ice and heat transfer can also help with everyday activities, such as preparing cold drinks, packing coolers for picnics, or even improving energy efficiency in your home.
Video : Why does ice float on water?
Conclusion: Ice Isn’t the Coolant—It’s the Heat Transfer
So, the next time you drop ice into your drink and wonder why it seems to cool down, remember this: the water is actually heating up the ice, not the other way around. It’s a fascinating and simple demonstration of how energy flows from hot to cold, and how materials like ice absorb and release heat.
By understanding this process, you can have a deeper appreciation of how energy transfer works in both natural and engineered systems. It’s not magic—it’s science. And next time you’re enjoying an iced beverage, you’ll know exactly what’s going on behind the scenes!


