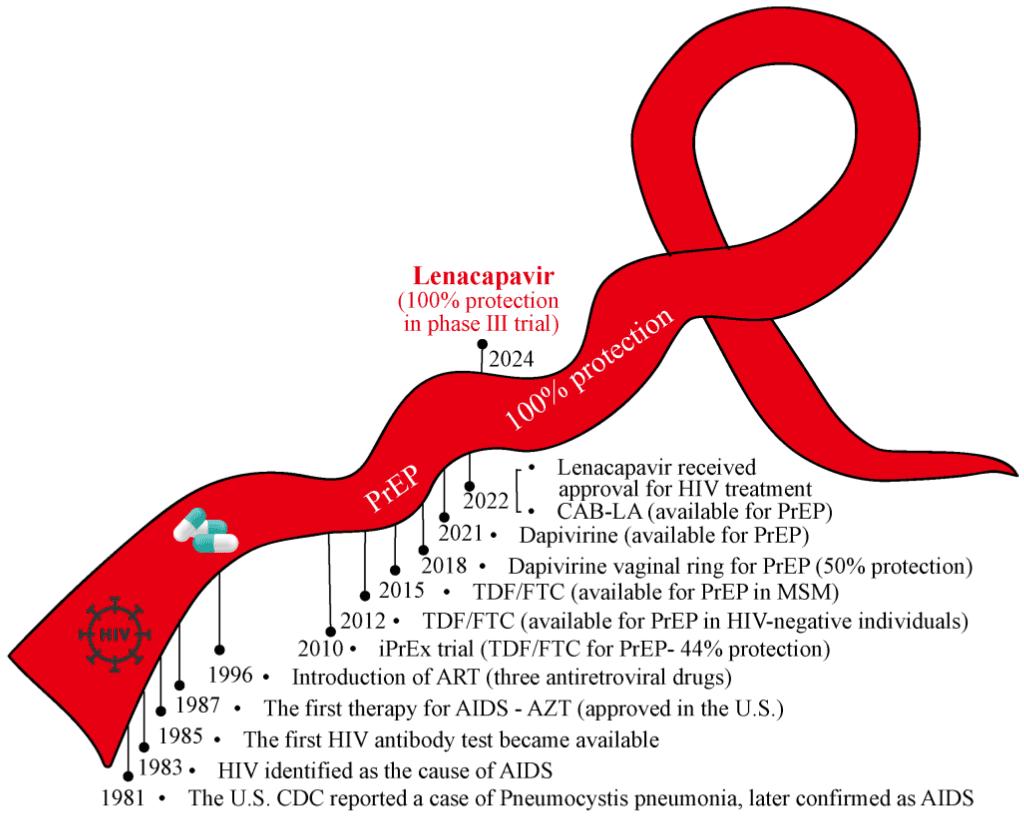
The fight against HIV has seen numerous advancements over the decades, but 2024 marked a significant breakthrough with the emergence of Lenacapavir. This groundbreaking injectable drug has achieved 100% efficacy in clinical trials, earning it the title of “Breakthrough of the Year” by Science journal.
Unlike traditional HIV treatments that require daily medication, Lenacapavir provides six months of protection with a single injection. This revolutionary approach has the potential to transform global HIV prevention strategies, offering hope to millions worldwide. But what makes Lenacapavir so effective, and how does it compare to existing treatments? Let’s explore.
How Does Lenacapavir Work?
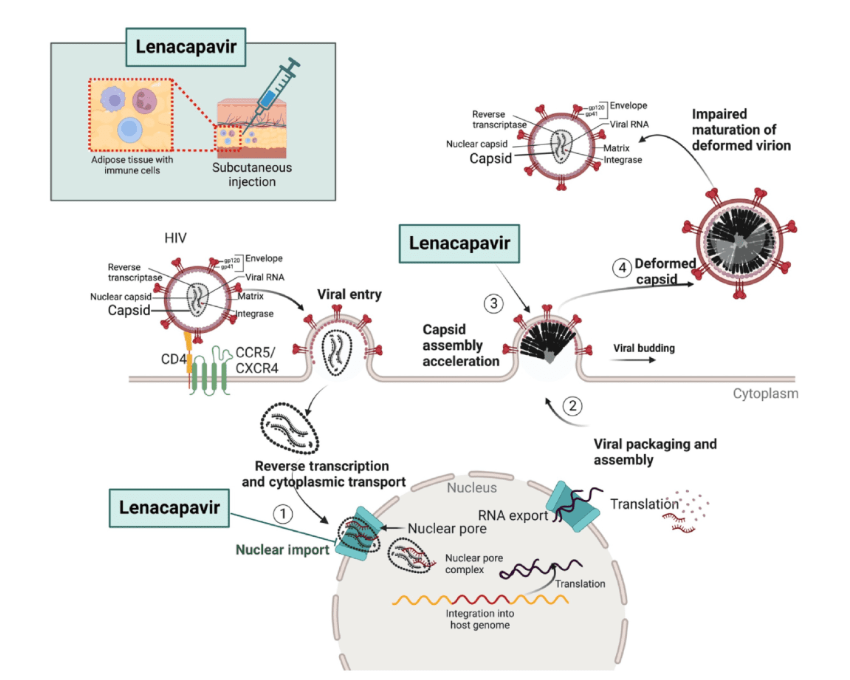
Lenacapavir targets the HIV capsid, the protein shell that protects the virus’s genetic material. Unlike conventional treatments that focus on blocking viral enzymes, this drug reinforces and disrupts the capsid, preventing HIV from:
✔ Replicating inside host cells
✔ Integrating into human DNA
✔ Spreading throughout the immune system
By interfering with these essential processes, Lenacapavir stops HIV at its source, preventing infection in the first place.
Lenacapavir’s Game-Changing Dosage Schedule
One of the biggest barriers to effective HIV prevention has been adherence to daily medication. Traditional pre-exposure prophylaxis (PrEP) requires users to take a pill every day, which can be difficult for many people. Injectable alternatives like Cabotegravir have improved convenience, reducing dosing frequency to once every two months.
However, Lenacapavir takes it even further—requiring only two injections per year to maintain full protection. This biannual dosing schedule marks a massive improvement in:
✔ Convenience – No need to remember daily pills
✔ Effectiveness – No risk of missed doses leading to infection
✔ Access – More suitable for communities with limited healthcare services
Researchers at Gilead Sciences, the pharmaceutical company behind Lenacapavir, are also investigating ways to extend the drug’s protection to a full year per dose, which would revolutionize HIV prevention even further.
Video : 100% Effective HIV Drug; A New Era in HIV Prevention. Lenacapavir Breakthrough
Clinical Trials: The Numbers Speak for Themselves
In 2024, two major clinical trials demonstrated Lenacapavir’s unprecedented effectiveness:
Study on Women and Adolescents in Africa
✔ Conducted in South Africa and Uganda
✔ Included over 5,000 transgender and cisgender female participants
✔ Zero cases of HIV infection among those who received Lenacapavir—100% efficacy
Multinational Study
✔ Conducted in South America, Asia, Africa, and the U.S.
✔ Included over 2,000 participants of different genders and risk groups
✔ Achieved 99.9% efficacy, with only two cases of infection recorded
In the PURPOSE-2 trial (2024), Lenacapavir reduced HIV risk by 96% when administered every six months. Among 2,180 participants, only two infections occurred, compared to nine infections in the 1,087 participants taking daily oral PrEP (Tenofovir/Emtricitabine).
What This Means for HIV Prevention
Health experts have hailed these results as “unprecedented”, positioning Lenacapavir as one of the most promising HIV prevention tools ever developed.
How Lenacapavir Compares to Other HIV Treatments
Lenacapavir represents a major leap forward in HIV prevention compared to existing treatment options.
| Feature | Lenacapavir | Oral PrEP (Tenofovir/Emtricitabine) | Injectable PrEP (Cabotegravir) |
|---|---|---|---|
| Dosing Frequency | Every 6 months | Daily pills | Every 2 months |
| Efficacy | 100% in some trials | ~74-99% | ~88-92% |
| Ease of Use | High – Only two injections per year | Low – Requires strict daily adherence | Moderate – Bi-monthly injections |
| Target Mechanism | Capsid Inhibition | Reverse Transcriptase Inhibition | Integrase Inhibition |
Clearly, Lenacapavir outperforms existing options in terms of efficacy, convenience, and protection—making it the new gold standard in HIV prevention.
Challenges to Widespread Adoption
Despite its remarkable success, several barriers could delay Lenacapavir’s global accessibility:
1. Cost and Affordability
✔ Gilead Sciences has partnered with manufacturers to produce generic versions in 120 developing nations.
✔ Pricing remains unclear in middle-income countries, where access to cutting-edge drugs is often limited.
2. Healthcare Infrastructure
✔ Some regions lack medical facilities capable of administering biannual injections.
✔ Storage and distribution logistics need improvement for widespread use.
3. Social and Cultural Stigma
✔ HIV-related stigma remains a major barrier, preventing people from seeking preventive treatment.
✔ Educational campaigns are needed to raise awareness and encourage uptake.
Video : Breakthrough in HIV prevention with lenacapavir
The Future of HIV Prevention: Is a Vaccine Still Necessary?
While Lenacapavir is a major milestone, it’s not a cure—and it does not eliminate the need for an HIV vaccine.
A vaccine would provide lifelong protection, eliminating the need for ongoing treatments. However, until that day comes, Lenacapavir remains the most effective HIV prevention tool available today.
What the WHO Says About Lenacapavir
The World Health Organization (WHO) has expressed strong support for Lenacapavir, calling its clinical trial results “highly encouraging”.
WHO is currently developing new guidelines to incorporate Lenacapavir into global public health programs, with a focus on:
✔ Expanding access in high-risk populations
✔ Reducing new HIV infections in low-income regions
✔ Ensuring affordable pricing and widespread availability
Conclusion
Lenacapavir isn’t just another HIV treatment—it’s a game-changer in global HIV prevention. With 100% efficacy in some trials, only two injections per year, and a radically new mechanism of action, this breakthrough drug could reshape the HIV epidemic forever.
However, to fully realize its potential, governments, pharmaceutical companies, and global health organizations must work together to overcome cost, accessibility, and stigma-related barriers.
We are closer than ever to a future without HIV—and Lenacapavir is leading the way.


