Severe retinal dystrophy associated with AIPL1 gene deficiency has long been a significant challenge in ophthalmology. This condition, which affects young children, leads to rapid and irreversible vision loss from birth. Until now, there have been no effective treatments available. However, a new gene therapy trial has shown promising results in restoring partial vision and slowing disease progression in affected children.
This groundbreaking first-in-human interventional study aims to determine the safety and effectiveness of rAAV8.hRKp.AIPL1, a recombinant adeno-associated viral vector designed to replace the faulty AIPL1 gene and potentially restore retinal function.
Understanding AIPL1-Associated Retinal Dystrophy
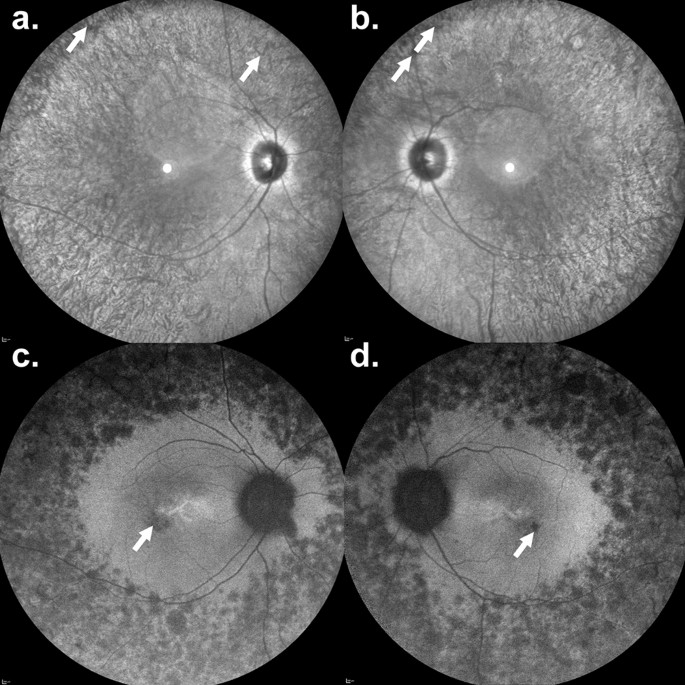
AIPL1-related retinal dystrophy is a rare, inherited condition that affects the photoreceptor cells in the retina. These cells play a crucial role in detecting light and transmitting visual signals to the brain. Without functional AIPL1 protein, photoreceptor cells deteriorate rapidly, leading to severe visual impairment from infancy. Symptoms include:
- Congenital nystagmus (uncontrolled eye movements)
- Reduced pupil response to light
- Severely impaired vision from birth
- Progressive photoreceptor cell degeneration
This condition is categorized under Leber Congenital Amaurosis (LCA) and is estimated to affect 1 to 3 in 100,000 individuals worldwide. Current treatment options are limited, with no approved therapies for AIPL1-related retinal dystrophy—until now.
Breakthrough in Gene Therapy: The Clinical Study
This study, conducted in the United Kingdom, involved four children aged 1.0 to 2.8 years, all diagnosed with biallelic disease-causing mutations in AIPL1. The research team used a recombinant AAV8 vector to deliver a functional AIPL1 gene directly into the retina via subretinal injection.
Video : What Is Retinal Gene Therapy?
Key Aspects of the Study
- Study Type: Open-label, first-in-human, interventional trial
- Participants: Four children with severe AIPL1-related retinal dystrophy
- Treatment: Subretinal injection of rAAV8.hRKp.AIPL1 in one eye
- Follow-up Duration: 3.0 to 4.1 years post-treatment
- Assessments: Visual acuity tests, functional vision evaluation, retinal imaging, and safety monitoring
The goal was to evaluate safety, monitor improvements in visual function, and determine whether early intervention could preserve retinal structure.
Remarkable Improvements in Vision and Retinal Function
Before receiving the treatment, all participants exhibited light perception only (logMAR 2.7), indicating severe vision impairment. However, the results post-treatment were extraordinary:
- Improved Visual Acuity: At the final follow-up, treated eyes achieved a mean of 0.9 logMAR (range: 0.8–1.0), demonstrating substantial vision improvement.
- Untreated Eyes Deteriorated: The untreated eyes became unmeasurable, reinforcing the effectiveness of gene therapy in preserving vision.
- Increased Cortical Response: Visual evoked potential (VEP) testing confirmed that the visual cortex responded better to stimuli in treated eyes.
- Structural Retinal Preservation: Handheld optical coherence tomography (OCT) showed better retinal thickness and lamination of the outer retina in treated eyes.
These findings indicate that gene therapy not only improved vision but also helped slow down retinal degeneration, a crucial factor in long-term disease management.
Safety Profile and Observed Side Effects
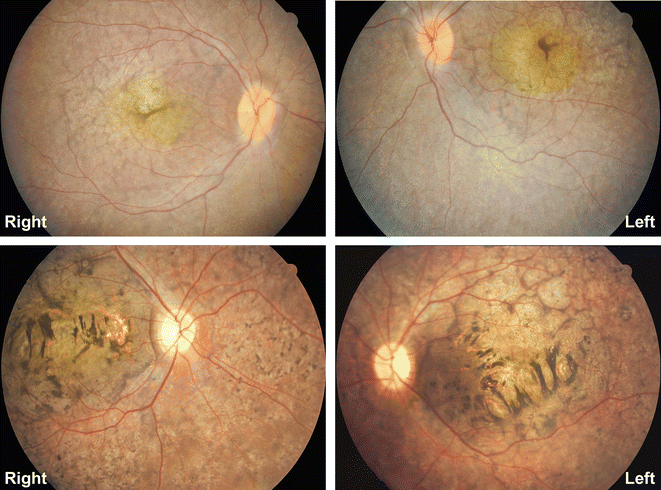
Ensuring patient safety was a top priority in this study. Key measures included:
- Oral Prednisolone Administration: Used to minimize inflammation post-treatment.
- Frequent Monitoring: Patients underwent detailed ocular examinations, including slit-lamp biomicroscopy, fundus imaging, and retinal scans.
- Minimal Side Effects:
- One child developed cystoid macular edema, which was managed effectively.
- No cases of retinal detachment or other serious complications were observed.
Overall, the safety profile was excellent, with no major adverse effects, confirming that gene therapy is a viable and safe option for young children with severe retinal dystrophy.
How Does Gene Therapy Work?
Mechanism of rAAV8.hRKp.AIPL1 Therapy
- Delivery of the AIPL1 Gene: The therapy uses an adeno-associated virus (AAV8) vector to introduce a healthy copy of the AIPL1 gene into retinal cells.
- Photoreceptor Rescue: The functional AIPL1 protein helps stabilize photoreceptor function, restoring some vision and preventing further degeneration.
- Long-Term Benefits: Unlike traditional treatments, gene therapy aims for long-lasting effects, potentially preserving vision for decades.
This strategy aligns with existing FDA-approved AAV gene therapies, such as voretigene neparvovec (Luxturna), used for RPE65-related retinal dystrophy.
Implications for Future Research and Treatment
A Paradigm Shift in Retinal Disease Management
This study sets a new precedent for treating genetic retinal dystrophies. The results suggest that:
- Early intervention is crucial to maximizing the benefits of gene therapy.
- The success of AIPL1 gene therapy could lead to similar treatments for other genetic eye diseases.
- More clinical trials are needed to refine dosing, delivery techniques, and long-term safety profiles.
Video : Genetic Counseling: Retinal Dystrophy Clinic at UPMC
Expanding Treatment to More Patients
Given the positive outcomes, there is a strong scientific and ethical argument for making AAV-based gene therapy widely available. This requires:
- Regulatory approval from health authorities
- Long-term follow-up studies to track effectiveness
- Development of affordable treatment options to ensure accessibility
Conclusion: A Major Step Toward Treating Blindness in Children
The first-in-human gene therapy trial for AIPL1-related severe retinal dystrophy has delivered promising results, offering new hope for children born with this condition. By improving visual acuity, preserving retinal structure, and preventing disease progression, rAAV8.hRKp.AIPL1 therapy represents a groundbreaking advancement in ophthalmology.
As gene therapy continues to evolve, we are witnessing a new frontier in medicine—one where genetic blindness may no longer be a life sentence. This historic milestone paves the way for a future where inherited retinal diseases can be effectively treated, restoring vision and transforming lives.